UAlbany Chemist Awarded $2 Million to Advance Computational Simulations for RNA Analysis
By Erin Frick
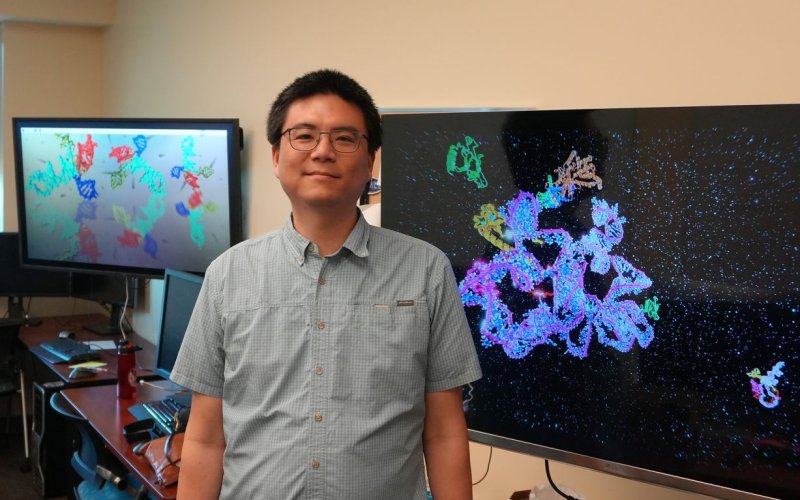
ALBANY, N.Y. (May 27, 2025) — Alan Chen, associate professor in chemistry and the RNA Institute, was awarded $2 million from the National Institute of General Medical Sciences, part of the National Institutes of Health (NIH). The grant will fund Chen’s lab over the next five years as they work to develop new ways to model the structure and function of RNA in 3D at the atomic level. The research aims to advance RNA-based biosensing and nanotechnology and will help solve longstanding puzzles in RNA structural biology.
Many diseases, including coronaviruses, genetic disorders and various forms of cancer, are caused by anomalies rooted in ribonucleic acids (RNAs) — the “messenger molecules” which among other roles, determine which genes get turned on and off inside cells. In order to treat or cure any RNA disease, we first need to understand the molecular cause.
“RNAs are dynamic and can self-assemble into intricate structures that are essential to cellular functions,” said Chen. “These features hold tremendous potential for solving important medical challenges, such as developing more precise diagnostic tools and creating new approaches to treating rare diseases. However, the same features that lend RNAs promise as a revolutionary biomedical tool also make them challenging to study.”
The primary traditional method for studying RNA molecules is cryo-electron microscopy. This method involves freezing a biological sample so it can be imaged using an electron beam.
“While this technique is excellent for creating static images of molecules, it fails to capture RNA's dynamic nature, including how it moves, folds and changes over time,” said Chen. “My lab is working to address these limitations by designing computational simulations that model RNA, including its movement, base pair formation and interactions with other molecules. By enabling more precise understanding of how RNA behaves, these simulations can inform experimental studies aimed at developing new biomedical tools and treatments.”
Molecules in Motion
At room temperature, RNA is constantly moving. This “dance” is an intrinsic feature of RNA and is what allows it to fold into shapes that either facilitate healthy cell function or contribute to disease. As RNA moves and folds, it is also interacting with other molecules and structures in the cell that have their own interconnected processes and pathways. This makes for a very active and complicated environment to examine closely, yet this level of understanding is essential to solving questions around the origin of RNA diseases.
“RNA simulations can reveal dynamic molecular behaviors that traditional ‘snapshot’ imaging cannot,” said Chen. “Our simulations allow us to identify missing biological components and create hypothetical scenarios in order to predict molecular interactions and outcomes that would otherwise be impossible to study.”
Designing ‘Minimal Models’
RNAs are made up of chains of nucleotide bases that can be sequenced. Chen’s team uses these sequences as “inputs” to feed mathematical models that produce visual representations of RNA structures.
“Similar to how you might add players and environmental features into a fantasy world in a video game, we can add molecules into our simulations to interact with a particular RNA,” said Chen. “By setting parameters that represent cellular activity, we can create dynamic visual simulations that help us make predictions about the molecular processes that cause disease. We can also ask questions like how a new small molecule drug might impact a ‘toxic’ RNA. Once we’ve identified promising avenues of study using simulations, we can test them experimentally using living cells.”
“It is also important to note that cells encapsulate many molecular pathways and processes, and not all of these are relevant to understanding a particular disease,” said Chen. “Instead, our approach focuses on designing what are called ‘minimal models’ to simplify the picture and narrow in on the most important mechanisms that could inform our understanding of a certain disease.”
Case Study: Myotonic Dystrophy
Chen works with collaborators across UAlbany’s RNA Institute to develop computer simulations unique to their particular research questions. These include projects addressing Zika virus, SARS-COV2, Dengue virus, Hepatitis C and myotonic dystrophy (DM), a rare muscular disease wherein an RNA-based anomaly causes certain nucleotides to repeat out of control. Myotonic dystrophy is a major research focus at the RNA Institute, with Professor Andy Berglund at the helm.
“The Berglund lab cultivates muscle cells taken from myotonic dystrophy patients which can be used to test our proposed therapies and determine whether a new small molecule could potentially rescue muscle function in diseased cells,” said Chen. “My lab creates computer simulations that include the toxic RNAs that cause myotonic dystrophy — modeling the shapes they make, what proteins they bind to — together with proposed small molecule drugs. By simulating what happens when the drug is introduced to the RNA, we can determine which compounds show promise and should be tested on actual muscle cells.”
The simulation video above models a key mechanism in the myotonic dystrophy disease system. This visualization helps show what happens when “muscleblind-like” (MBNL) protein is prevented from properly splicing RNA in the muscle cells of people with myotonic dystrophy. This gives rise to the disease’s signature repeat expansion and toxic RNAs which cause symptoms of the disease.
“Although there is still much to learn before this technology could be used to treat diseases in people, our work now is laying critical groundwork and addressing key technical challenges to enable future therapies, and potentially even cures, across RNA diseases,” Chen said.