Two Cellular Scientists Rewarded as ‘Early Stage Investigators’
ALBANY, N.Y. (July 22, 2021) — Two scientists making strides in their independent research have received robust financial encouragement from NIH’s National Institute of General Medical Sciences (NIGMS) to make critically important breakthroughs in the effort to reveal cellular causes of disease and to create novel therapeutics.
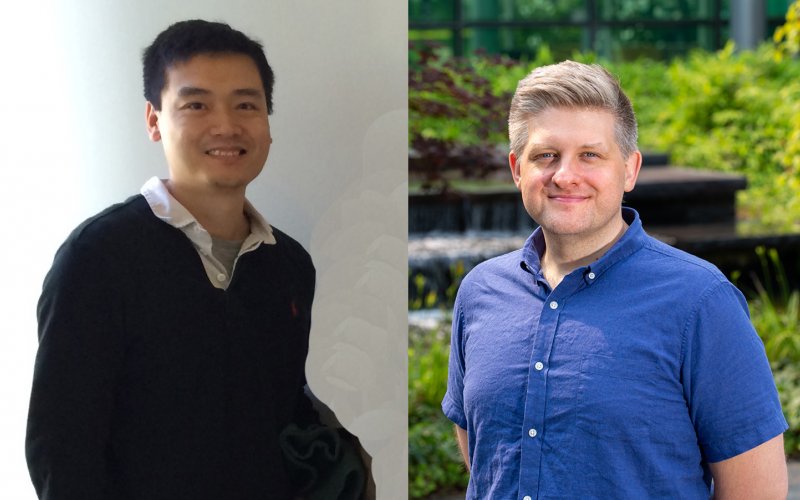
Assistant Professor Morgan Sammons of Biology and Associate Professor Qiang Zhang of Chemistry each won a 5-year NIGMS Maximizing Investigators' Research Award (MIRA) for Early Stage Investigators — Sammons, for his team’s project “Defining cis-regulatory networks controlling a core stress response,” and Zhang, for his lab’s “The chemical approach towards homogenous glycoprotein preparation and evaluation.”
The goal of MIRA is to increase the efficiency of NIGMS funding by providing investigators with greater stability and flexibility, thereby enhancing scientific productivity and the chances for significant discovery. Two RNA Institute scientists who have conducted major NSF and NIH-funded studies after receiving MIRAs are Ken Halvorsen (2017) and Alan Chen of Chemistry (2019).
“I’m very excited about these MIRA awards, particularly for early-stage investigators, who can really get a terrific boost that helps them successfully launch their independent careers,” said Distinguished Professor Marlene Belfort, senior advisor to the RNA Institute.
“But at any career stage,” she said, “the MIRA provides a stable funding source that allows the PI the luxury to do a balanced risk-return tradeoff in their research, without worrying where the next year’s funding will come from. And, as we all know, it’s the high-risk high-reward investigations that often lead to scientific breakthroughs. So, my enthusiastic congratulations to Drs. Sammons and Zhang!”
The Sammons project, funded at $1.765 million, focuses on cellular response to DNA damage and the transcription factor p53, a central hub within the DNA damage gene-regulatory network. Misregulation of p53 activity is directly implicated in numerous deleterious human disorders, such as premature aging, cancer, neuro-developmental diseases and infertility, said Sammons. Multiple therapeutic approaches also are strongly inhibited by the p53 pathway.
To date, researchers lack key insights into how p53 controls cell fate decisions after exposure to DNA damage. That is why, through MIRA funding, Sammons’ team is engaged in a vigorous examination of the complex gene networks controlled by p53. Ultimately, this work will launch a long-term research program focused on investigation into the molecular mechanisms underlying transcription factor regulation of cell fate decisions and human disease.
Zhang’s project, funded at $1.839 million, seeks to uncover the now-unknown function of cellular and infectious prion protein. The research is relevant to public health because cyclic peptides and glycoproteins exist ubiquitously in pharmaceuticals and biological medical products termed biologics.
Despite significant advances in chemical studies of protein preparation, homogenous membrane glycoproteins are difficult to obtain. Zhang’s objective is to develop a class of efficient peptidyl coupling protocols utilizing strained molecules for the construction of previously unattainable targets, such as constrained cyclic tetrapeptides and membrane prion protein.
“Upon conclusion,” said Zhang, “we will provide a powerful tool for protein function elucidation and investigation that has not been available to scientists.”
NIGMS awards for both established and early stage investigators supports basic research that increases the understanding of biological processes and lays a foundation for advances in disease diagnosis, treatment and prevention.
“I am incredibly grateful to the NIH for supporting my team’s research and to my former trainees who did so much of the work to get us to this point,” noted Sammons.
“This award will provide my current and future students an opportunity to learn novel genetic, genomic and computational techniques and help us all better understand how individual cells survive and thrive after exposure to acute and chronic stress. I am excited about the award and the flexibility it provides us to explore new directions as we obtain new data.”