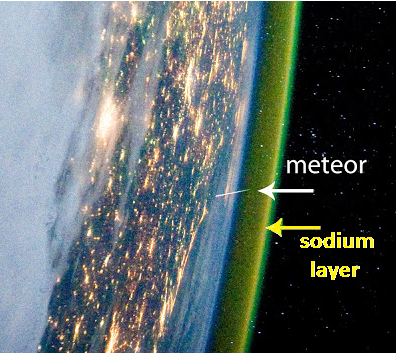
| 1929 | Sodium (Na) layer discovered by Vesto Melvin Slipher |
| 1939 | Chapman proposes theory to explain layer |
| 1960's | Routine observations begin |
| 1969 | Lidar observations begin |
| 1976 | Iron (Fe) layer detected |
| 1985 | First extensive laboratory studies of Na atom chemistry relevant to atmosphere |
| 1989 | Lidar observations of iron layer begin |