John T. Schmidt
Professor, Biological Sciences
University at Albany
State University of New York
1400 Washington Avenue
Albany, NY 12222
Office: Bio 121
Phone: 518-442-4309
FAX: 518-442-4767
EMail: [email protected]
|  |
Research Interests:
- Developmental Neurobiology
- Formation of Retinotopic Maps
- Activity-Driven Synaptic Refinement
- Development and Regeneration of Retinotectal
Synapses
- Actin, Myosin and Growth Cone Motility
- Myosin Light Chain Kinase, Rho kinase
Recent Publications:
- Wolpaw, J.R., Schmidt, J.T. and Vaughan, T.M. (1991) Activity-driven CNS changes in learning
and development. (Proceedings of a conference held in May 1990) Vol. 627 of the Annals of the
New York Academy of Sciences.
- Jian, X., Szaro, B. and Schmidt, J.T. (1996) Myosin light chain kinase: Expression in neurons
and upregulation during axon outgrowth. J. Neurobiology 31: 379-391.
- Jian, X., Hidaka, H. and Schmidt, J.T. (1994) Kinase requirement for retinal growth cone
motility. J. Neurobiology 25: 1310-1328.
- Schmidt, J.T., Schmidt, R., Lin, W., Jian, X. and Stuermer, C.A.O. (1991) Ependymin as a
substrate for outgrowth of axons from cultured explants of goldfish retina. J. Neurobiology
22:40-54.
- Benowitz, L.I., and Schmidt, J.T. (1987) Activity-dependent sharpening of the regenerating
retinotectal projection in goldfish: relationship to the expression of growth-associated proteins.
Brain Research 417: 118-126.
- Schmidt, J.T. and Buzzard, M. (1990) Activity-driven sharpening of the regenerating retinotectal
projection: Effects of blocking or synchronizing activity on the morphology of individual
regenerating arbors. J. Neurobiology 21: 900-917.
- Schmidt, J.T. (1990) Long-term potentiation and activity-dependent retinotopic sharpening in the
regenerating retinotectal projection of goldfish: Common sensitive period and sensitivity to
NMDA blockers. J. Neuroscience 10: 233-246.
- Schmidt, J.T. and Buzzard, M. (1993) Activity-driven sharpening of the retinotectal projection in
goldfish: Development under stroboscopic illumination prevents sharpening. J. Neurobiology 24:
384-399.
- Schmidt, J.T. (1994) C-kinase manipulations disrupt activity-driven retinotopic sharpening in
regenerating goldfish retinotectal projection. J. Neurobiology 25: 555-570.
Statement on Research Program:
Research in my laboratory currently centers on two areas: the activity-driven refinement of
retinotopic connections in the visual system and the role of myosin light chain kinase in regulating
actin-myosin based growth cone motility.
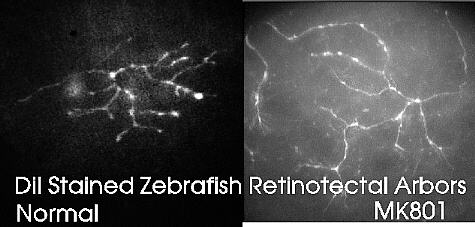
During development or regeneration of the retinotectal projection, a rough retinal map is first
formed and then sharpened through an activity-driven sharpening of synaptic connections. Each
retinal axon forms exuberant connections and later prunes them to the final precise set. The same
mechanism also gives rise to eye-specific termination zones and to the segregation of projections
from ganglion cells of the same receptive field type. Using pharmacological agents to block
NMDA receptors prevents this retinotopic sharpening and also blocks the induction of long-term
potentiation (LTP) at these synapses which may be the first step in synaptic stabilization. C-kinase
activation is also necessary for both, giving direct parallels between development and learning.
Current studies focus on vital staining of developing arbors in zebrafish larvae to see the effects
of pharmacological agents in time-lapse in vivo, and on electrophysiological studies of
transmission in slices and
cultures.
The second area focuses on the molecular mechanisms regulating growth cone motility. Both
actin and myosin are present in growth cones within the lamellipodia and filopodia, and
actin-myosin force generation elsewhere is controlled by myosin light chain kinase (MLCK) via
the phosphorylation of the myosin light chain. We have shown that pharmacological inhibitors of
MLCK stop growth cone motility, collapse lamellipodia and filopodia, and deplete filamentous
actin. More recently we have cloned the MLCK gene from goldfish, shown for the first time that
it is expressed in neurons with in situ hybridization, and also that it is prominently upregulated in
retinal ganglion cells during the regeneration of their axons. We have made inhibitory peptides
coupled to fatty acid that can cross the membrane and inhibit MLCK, and they verified the results
produced by the pharmacological inhibitors. Similarly constructed peptide inhibitors of protein
kinase C do not produce these effects. We can also produce many of the same effects with the
myosin ATPase inhibitor, butanedione monoxime. Finally we have made a peptide specific
polyclonal antibody for localization of MLCK.
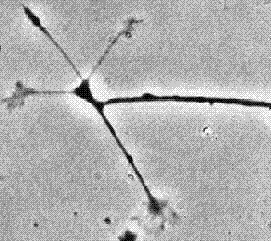 Growing axon with multiple growth cones.
Growing axon with multiple growth cones.
|




 Growing axon with multiple growth cones.
Growing axon with multiple growth cones.